VALENCE
Lithium Battery Supplier-
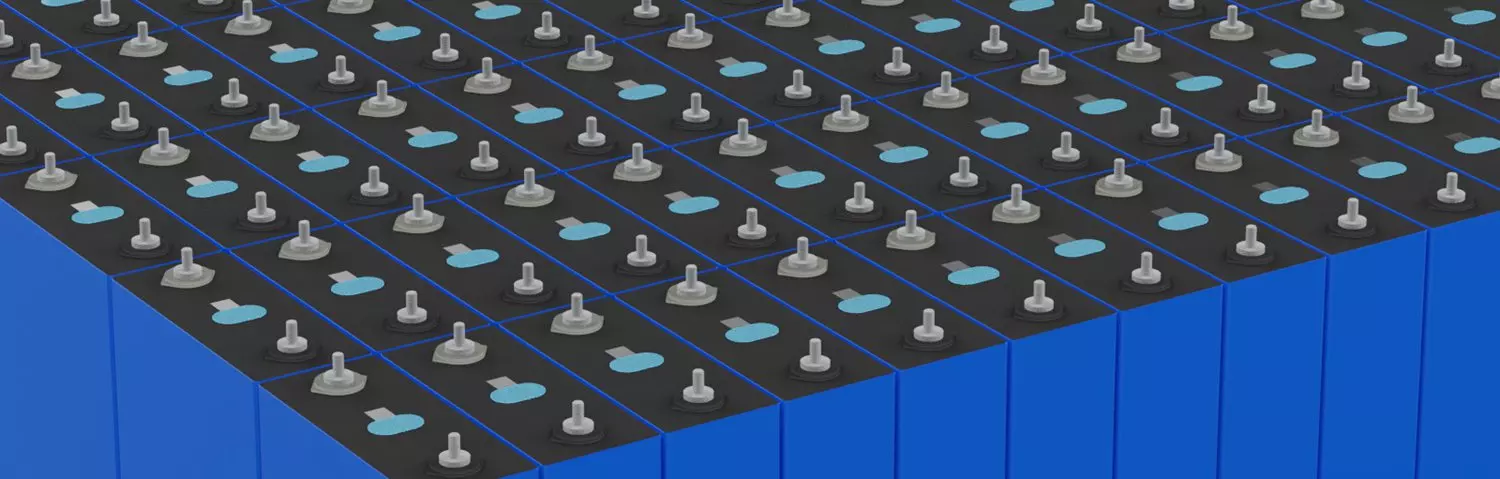
Gotion 340Ah LiFePO4 Lithium Battery Cell
Ship from China US$86.00
-
CATL 3.2V 120Ah LiFePO4 Lithium Battery Cell
Ship from China Get Price
-
CATL 3.2V 271Ah LiFePO4 Lithium Battery Cell
Ship from China Get Price
-
GanFeng 3.2V 100Ah LiFePO4 Lithium Battery Cell Wholesale
Ship from China Get Price
-
HiGee 3.2V 120Ah LiFePO4 Lithium Battery Cell Supplier
Ship from China Get Price
-
HiStar 3.2V 100Ah LiFePO4 Lithium Battery Cell Distributor
Ship from China Get Price
-
Solution Battery Module -
Solution LTO Cells for Car Audio
Valence: The Key to Elemental Understanding In the realm of chemistry, valence holds a crucial position, serving as the foundation for defining the properties and behaviors of elements. Valence, also known as valency, refers to the combining power of an element, specifically the number of electrons an atom shares or gains to form chemical bonds with other atoms. This fundamental concept allows chemists to categorize and predict the reactions and characteristics of various elements, revolutionizing our understanding of the molecular world. From a historical perspective, the concept of valence has undergone significant refinement, with pioneer scientists such as Irving Langmuir and Gilbert Newton Lewis contributing to its development. Langmuir's introduction of the octet rule, which posits that atoms tend to gain or lose electrons to achieve a stable configuration of eight electrons in their outermost energy level, laid the groundwork for modern valence theory. Similarly, Lewis's concept of electron pairs and the sharing of electrons in chemical bonds further clarified the nature of valence. In modern chemistry, valence plays a vital role in understanding the properties and behavior of elements, particularly in their ability to form compounds. The combination of valence electrons in an atom determines the type of bonds it can form, whether covalent, ionic, or metallic. This, in turn, influences the physical and chemical properties of the resulting compounds, such as their reactivity, melting points, and optical properties. As a fundamental principle in chemistry, valence has far-reaching implications for various fields, including materials science, biology, and environmental science. By grasping the concept of valence, scientists can better understand the interactions between atoms and molecules, ultimately driving innovations in fields such as nanotechnology, pharmacology, and sustainable energy production. In conclusion, valence is a cornerstone of chemistry, connecting the properties and behaviors of elements to their fundamental electronic structure. Through its continued refinement and application, the concept of valence empowers chemists to unlock the secrets of the molecular world, fostering breakthroughs in various disciplines and driving human progress.
 Europe Warehouse
Europe Warehouse