BINAXNOW
Lithium Battery Supplier
Lithium Battery Supplier, LiFePO4 Battery Wholesale. One Stop Services.
-
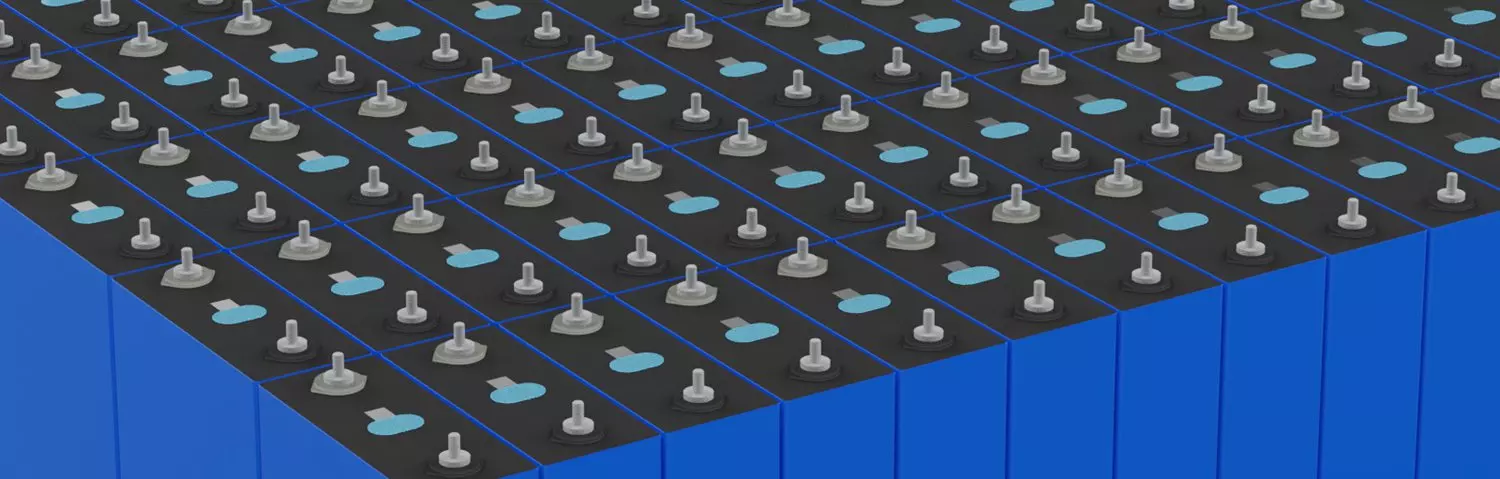
Solution Battery Module -
Solution Energy Storage Battery
BinaxNOW is a groundbreaking, at-home COVID-19 antigen test that empowers individuals to take control of their health and well-being. Developed by Abbott, a renowned healthcare company, this innovative self-test provides quick and accurate results, allowing users to detect the presence of SARS-CoV-2 in just 15 minutes. Utilizing a simple, fingerstick-based technology, the BinaxNOW test is designed for individuals aged 2 and above, providing an easy and non-invasive way to test for COVID-19. The test employs a proprietary antigent detection system, which combines Abbott's cutting-edge technology with a simple, user-friendly design. One of the most significant advantages of BinaxNOW lies in its ability to provide rapid results, making it an ideal solution for emergency situations, travel requirements, or simply to ensure the safety of oneself and others. The test consists of a single-use device, ensuring a clean and sanitary testing experience. With BinaxNOW, users can rest assured of high accuracy rates, with studies showing a sensitivity of 88.5% and specificity of 96.3% for detecting COVID-19. This means that individuals can rely on the results, knowing that the test will accurately detect the presence of the virus. Moreover, BinaxNOW has received emergency use authorization (EUA) from the US FDA, signifying its compliance with stringent regulatory standards. This ensures that the test has met rigorous testing and evaluation criteria, guaranteeing its effectiveness and safety. Get access to reliable, at-home COVID-19 testing with BinaxNOW. Order now and take control of your health, safely and efficiently. Keywords: BinaxNOW, At-home COVID-19 test, Abbott, COVID-19 antigen test, fingerstick-based technology, rapid results, high accuracy rate, emergency use authorization, US FDA.
 EU Stock
EU Stock Poland Stock
Poland Stock Ukraine Stock
Ukraine Stock  USA Stock
USA Stock AU Stock
AU Stock