ABBOTT COVID TEST
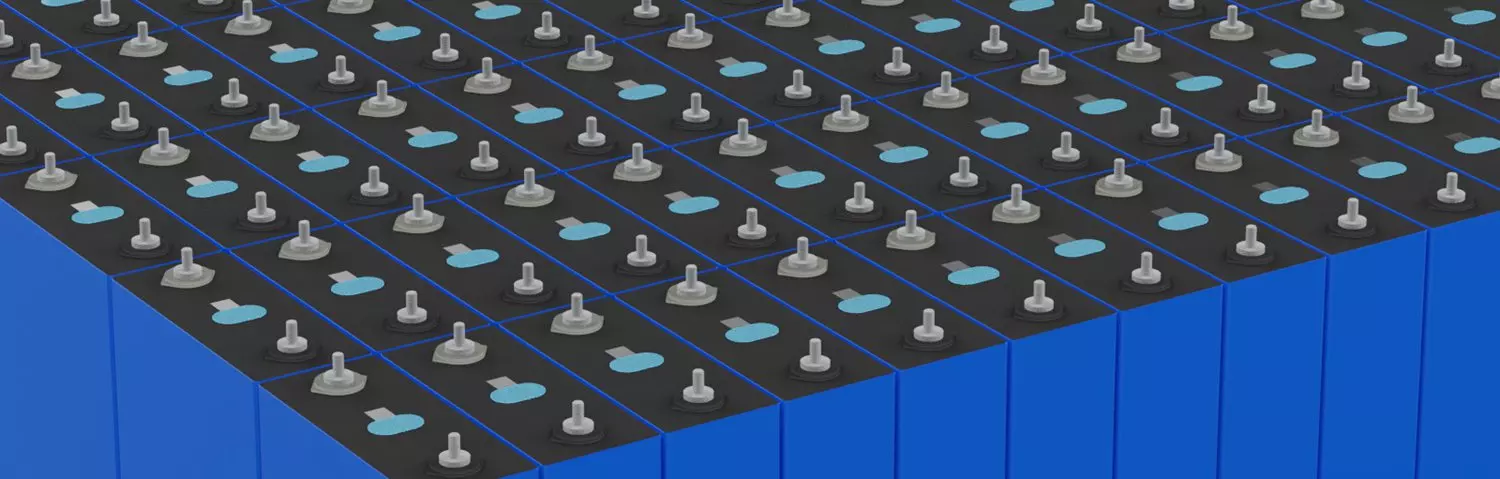
Lithium Battery Supplier
Lithium Battery Supplier, LiFePO4 Battery Wholesale. One Stop Services.
-
Solution Energy Storage Battery -
Solution Battery Active Equalizer
Abbot's cutting-edge COVID-19 test solutions have revolutionized the way we detect and manage the spread of the virus. At the forefront of innovation, Abbott's diagnostic products have consistently delivered accurate and reliable results, empowering healthcare professionals to make informed decisions and take swift action to control the outbreak. Abbott's COVID-19 tests are designed to detect the SARS-CoV-2 virus, diagnosing infections and monitoring the effectiveness of treatment and public health measures. With a focus on speed, sensitivity, and specificity, Abbott's tests have been broadly adopted by healthcare providers worldwide, including hospitals, clinics, and public health laboratories. One of the key advantages of Abbott's COVID-19 tests is their ability to detect the virus early in the infection, even before symptoms appear. This enables prompt identification and isolation of infected individuals, thereby reducing the transmission risk and mitigating the spread of the virus. Abbott's COVID-19 tests have been rigorously validated and are CE-IVD marked, ensuring compliance with the European Union's medical device regulations. This guarantees the highest standards of quality, safety, and performance, allowing healthcare professionals to trust the test results and make informed decisions. Moreover, Abbott's COVID-19 tests are designed to be user-friendly, reducing the complexity and variability associated with testing. This simplifies the testing process, making it more accessible and efficient for healthcare providers, ultimately enhancing patient care and public health outcomes. In response to the rapidly evolving COVID-19 landscape, Abbott continues to innovate and adapt its diagnostic solutions to meet the ever-changing needs of the healthcare community. With a commitment to innovation, Abbott is dedicated to delivering unparalleled testing solutions that support the global fight against COVID-19.
 EU Stock
EU Stock Poland Stock
Poland Stock Ukraine Stock
Ukraine Stock  USA Stock
USA Stock AU Stock
AU Stock